Introduction:
Gain/amplification of 1q21 (1q21+) is found in 40% of patients (pts) with newly diagnosed Multiple Myeloma (MM). There is increasing evidence that presence of gain of 1q21 [gain(1q21), 3 copies] or amplification of 1q21 [amp(1q21), ≥4 copies] are independent poor prognostic factors. A recent post-hoc analysis of the ICARIA-MM and IKEMA studies showed that the addition of monoclonal anti-CD38 antibody isatuximab in combination with pomalidomide-dexamethasone or carfilzomib-dexamethasone abrogated the negative impact of 1q21 on progression-free survival (PFS). However, the impact of 1q21+ has not been consistently assessed in daratumumab (Dara) exposed pts.
Methods:
We identified MM pts treated with Dara between Jan 2020 and Jan 2023 from the Australia-New Zealand and Asia-Pacific Myeloma and Related Diseases Registries. Pts who had interphase fluorescence in situ hybridization (FISH) testing were included. High-risk cytogenetics (HRCyto) was defined as the presence of t(4;14), t(14;16) or del(17p) by FISH, and standard risk cytogenetics (SRCyto) as pts without 1q21+ and HRCyto. Pt groups analysed were: (1) without 1q21+ vs with 1q21+ (2) SRCyto vs isolated 1q21+ vs HRCyto without 1q21+ vs HRCyto with 1q21+. Subgroup analyses performed include: (3) gain(1q21) vs amp(1q21). Overall survival (OS) and PFS from commencement of Dara were estimated using the Kaplan-Meier method. Comparisons of PFS and OS were performed using the log-rank test. Categorical data were compared using a chi-square test and continuous variables with a rank-sum test.
Results:
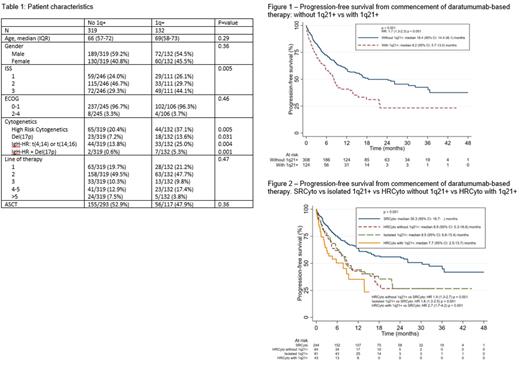
Of 654 pts who received Dara, 451 had interphase FISH testing performed and were included. There were 132 pts with 1q21+ [gain(1q): 23, amp(1q):14, gain/amp status unknown: 95] and 319 without. Median follow up was 15 months (95% CI 14-18). Median prior lines of therapy was 1 (IQR 1-2). Dara-based regimens used were: Dara monotherapy (17.7%), Dara-bortezomib-dex (54.6%), Dara-lenalidomide-dex (11.3%), Dara-pomalidomide-dex (DPd) (9.3%), Dara-carfilzomib-dex (DKd) (2.5%) and Dara-bortezomib-lenalidomide-dex (4.6%). There were no differences in age, gender, ECOG, line of therapy in which Dara was used, or rate of autologous stem cell transplantation between pts with 1q+ vs pts without (Table 1). Pts with 1q21+ had higher rates of other HRCyto (37.1% vs 20.4%; p=0.005) and ISS Stage 3 disease (44.1% vs 29.3%; p=0.005) vs those without 1q21+. There was no significant difference in overall response rate (65.9% vs 71.6%; p=0.32). Through univariate analyses, PFS for 1q21+ pts was significantly shorter than those without 1q21+ [median PFS 8.2 vs 18.4 months (HR 1.7, 95% CI 1.3-2.3; p<0.001)] (Figure 1). This difference was still significant when adjusted for ISS, the presence of other HRCyto and country of treatment (HR 1.61, 95% CI 1.17-2.22; p=0.004). Pts with 1q21+ with or without HRCyto, and those with HRCyto and no 1q21+ had inferior PFS vs SRCtyo (median PFS 7.7 vs 8.9 vs 8.5 vs 30.3 months; p<0.001) (Figure 2). Of the pts where 1q21+ copy number was available, OS was significantly poorer in pts with amp(1q) vs those with gain(1q) (median OS 22.6 vs 46.9 months; p=0.035). There was also a trend towards poorer PFS in pts with amp(1q) (median PFS 4.9 vs 10.5 months; p=0.096)
Conclusions:
1q21+ often co-exists with other HRCyto abnormalities. However, it remains an independent negative prognostic marker. The use of Dara does not appear to abrogate the negative impact of 1q21+ on PFS. Amp(1q21) potentially confers an even poorer outcome. This finding differs from an earlier retrospective study (Parrondo et al, Blood 2022), although <10% of patients in our study received DKd and DPd combinations. As such, more data on Dara in combination with carfilzomib and pomalidomide is needed.
Disclosures
Spencer:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Antengene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; IDP Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria. Mollee:Pfizer: Research Funding; Cilag: Research Funding; Janssen: Research Funding. Kim:Janssen, Amgen, BMS, Takeda, LG chem: Honoraria, Research Funding. McQuilten:Janssen-Cilag: Research Funding; CSL Behring: Research Funding; Bristol-Myers Squibb: Research Funding; Takeda: Research Funding; AstraZeneca: Research Funding; BeiGene: Research Funding; Gilead Sciences: Research Funding; Antengene: Research Funding; Roche: Research Funding. Quach:Sanofi: Consultancy, Other: receipt of study materials; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Leadership or fiduciary role; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: receipt of study materials; Leadership or fiduciary role, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: receipt of study materials, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal